Use of ATR-FTIR spectroscopy for studying adsorbed species and their interaction with adsorbent surfaces
DOI:
https://doi.org/10.19137/semiarida.2026(1).71-80Keywords:
ATR-FTIR, spectra, species, adsorption, kineticsAbstract
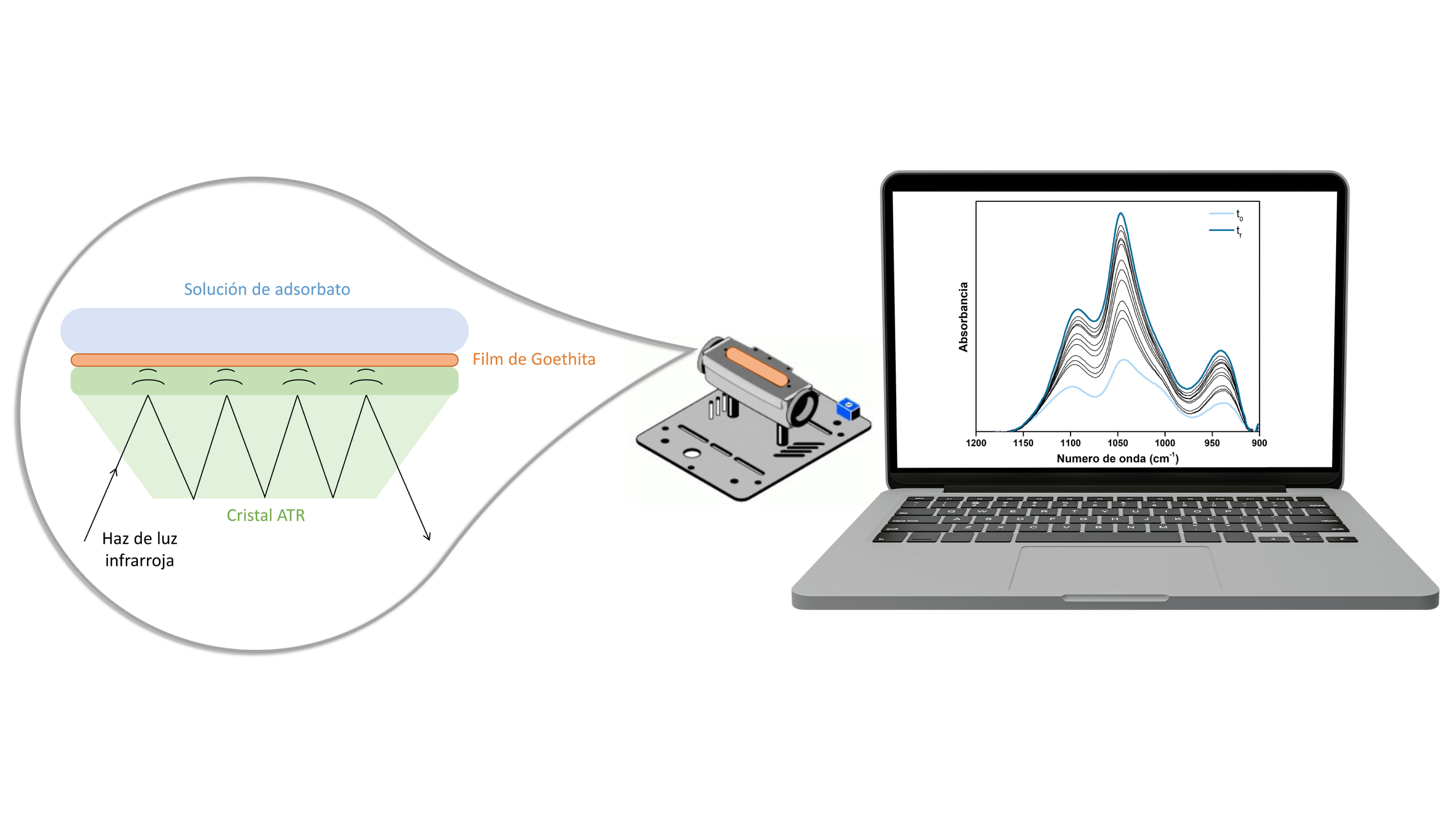
Attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR) is a fast, sensitive, versatile, and non-destructive technique capable of an in situ evaluation of the adsorption of ions and molecules at the liquid-solid interface. This technique allows for studies in aqueous media, a task impossible with conventional FTIR due to interferences caused by water signals. The molecular information acquired by this technique allows the determination of the adsorption mode, including, in some cases, conformational and structural changes of the adsorbed substance. Furthermore, under properly controlled conditions, ATR-FTIR spectroscopy can be used as a quantitative tool to evaluate adsorption and desorption kinetics, and adsorption under equilibrium conditions. In this work, as an example, the ATR-FTIR spectroscopy is used to study the adsorption-desorption kinetics of different anions on goethite. The kinetics were monitored in situ with precise control of pH, temperature, and adsorbate concentration throughout the experiment using a flow cell. The results obtained are comparable with those obtained by the batch technique, with the advantage of being a faster technique, allowing information to be obtained in the first minutes of reaction and monitoring the identity of the surface species throughout the reaction time.
Downloads
References
Andersson, P. O., Lind, P., Mattsson, A., & Österlund, L. (2008). A novel ATR-FTIR method for functionalised surface characterisation. Surface and Interface Analysis, 40(5), 623-626. https://doi.org/10.1002/sia.2661
Andrade, J., Pereira, C. G., de Almeida Junior, J. C., Viana, C. C. R., de Oliveira Neves, L. N., da Silva, P. H. F., Valenzuela Bell, M. J., & dos Anjos, V. C. (2019). FTIR-ATR determination of protein content to evaluate whey protein concentrate adulteration. LWT - Food Science and Technology, 99, 166–172. https://doi.org/10.1016/j.lwt.2018.09.079
Arroyave, J. M., Waiman, C. C., Zanini, G. P., & Avena, M. J. (2016). Effect of humic acid on the adsorption/desorption behavior of glyphosate on goethite. Isotherms and kinetics. Chemosphere, 145, 34–41. https://doi.org/10.1016/j.chemosphere.2015.11.082
Arroyave, J. M., Puccia, V., Zanini, G. P., & Avena, M. J. (2018). Surface speciation of phosphate on goethite as seen by InfraRed Surface Titrations (IRST). Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 199, 57–64. https://doi.org/10.1016/j.saa.2018.03.043
Arroyave, J. M., Avena, M., & Tan, W. (2022). The two‑species phosphate adsorption kinetics on goethite. Chemosphere, 304, 135045. https://doi.org/10.1016/j.chemosphere.2022.2755
Barbeş, L., Rădulescu, C., & Stihi, C. (2014). ATR-FTIR spectrometry characterisation of polymeric materials. Romanian Reports in Physics, 66(3), 765–777. https://www.researchgate.net/publication/256297992
Blum, M. M. & John, H. (2012). Historical perspective and modern applications of Attenuated Total Reflectance – Fourier Transform Infrared Spectroscopy (ATR-FTIR). Drug Testing and Analysis, 4, 298–302. https://doi.org/10.1002/dta.374
Carabante, I., Grahn, M., Holmgren, A., Kumpiene, J., & Hedlund, J. (2009). Adsorption of As(V) on iron oxide nanoparticle films studied by in situ ATR-FTIR spectroscopy. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 346, 106–113. https://doi.org/10.1016/j.colsurfa.2009.05.032
Dilshad, A., Taneez, M., Younas, F., Jabeen, A., Rafiq, M. T., & Fatimah, H. (2022). Microplastic pollution in the surface water and sediments from Kallar Kahar wetland, Pakistan: occurrence, distribution, and characterization by ATR-FTIR. Environmental Monitoring and Assessment, 194, Article 511. https://doi.org/10.1007/s10661-022-10171-z
Gentile, M. B., Gómez, S. R., Avena, M. J. & Luengo, C. V. (2025). The interaction of phenylphosphonic acid with the surface of goethite: Isotherms, kinetics, electrophoretic mobility and ATR-FTIR spectroscopy. Environmental Pollution, 371, 125938. https://doi.org/10.1016/j.envpol.2025.125938
Grdadolnik, J. (2002). ATR-FTIR Spectroscopy: Its advantages and limitations. Acta chimica Slovenica, 49, 631−642.
Harrick, N. J. (1967). Internal Reflection Spectroscopy. Wiley-Interscience, New York.
Hind, A. R., Bhargava, S. K. & McKinnon, A. (2001). At the solid/liquid interface: FTIR/ATR — the tool of choice. Advances in Colloid and Interface Science, 93, 91-114. https://doi.org/10.1016/S0001-8686(00)00079-8
Ismail, A. A., van de Voort, F. R. & Sedman, J. (1997). Fourier transform infrared spectroscopy: Principles and applications. Techniques and Instrumentation in Analytical Chemistry, 18, 93-139. https://doi.org/10.1016/S0167-9244(97)80013-3
Kazarian, S. G., & Chan, K. L. A. (2006). Applications of ATR-FTIR spectroscopic imaging to biomedical samples. Biochimica et Biophysica Acta (BBA) - Biomembranes, 1758(7), 858–867. https://doi.org/10.1016/ j.bbamem.2006.02.011
Luengo, C., Brigante, M., Antelo, J. & Avena, M. (2006) Kinetics of phosphate adsorption on goethite: Comparing batch adsorption and ATR-IR measurements. Journal of Colloid and Interface Science, 300, 511-518. https://doi.org/10.1016/j.jcis.2006.04.015
McQuillan, A. J. (2001). Probing Solid–Solution Interfacial Chemistry with ATR-IR Spectroscopy of Particle Films. Advanced Materials, 13, 1034-1038. https://doi.org/10.1002/1521-4095(200107)13:12/13<1034::AID-ADMA1034>3.0.CO;2-7
Mudunkotuwa, I. A., Minshid, A. A. & Grassian, V. H. (2014). ATR-FTIR spectroscopy as a tool to probe surface adsorption on nanoparticles at the liquid–solid interface in environmentally and biologically relevant media. Analyst, 139, 870-881. https://doi.org/10.1039/c3an01684f
Puccia, V., Luengo, C., & Avena, M. (2009). Phosphate desorption kinetics from goethite as induced by arsenate. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 348, 221–227. https://doi.org/10.1016/j.colsurfa.2009.07.026
Rahnemaie, R., Hiemstra, T. & van Riemsdijk, W. H. (2006). Inner- and outer-sphere complexation of ions at the goethite–solution interface. Journal of Colloid and Interface Science, 302, 403–416. https://doi.org/10.1016/j.jcis.2005.11.003
Rivera, D., Poston, P. E., Uibel, R. H. & Harris, J. M. (2000). In Situ Adsorption Studies at Silica/Solution Interfaces by Attenuated Total Internal Reflection Fourier Transform Infrared Spectroscopy: Examination of Adsorption Models in Normal-Phase Liquid Chromatography. Analytical Chemistry, 72, 1543-1554. https://doi.org/10.1021/ac990968h
Schmidt, M. P., Siciliano, S. D., & Peak, D. (2020). Spectroscopic Quantification of Inner- and Outer‑Sphere Oxyanion Complexation Kinetics: Ionic Strength and Background Cation Effect on Sulfate Adsorption to Hematite. ACS Earth and Space Chemistry, 4, 1765–1776. https://doi.org/10.1021/ acsearthspacechem.0c00149
Simonescu, C. M. (2012). Capítulo 2: Application of FTIR Spectroscopy in Environmental Studies. En M. A. Farrukh (Ed.), Advanced Aspects of Spectroscopy (pp. 49-84). InTech. http://dx.doi.org/10.5772/48331
Smith, B. C. (2011). Fundamentals of Fourier Transform Infrared Spectroscopy. CRC Press.
Yan, C., Huang, W., Ma, J., Xu, J., Lv, Q., & Lin, P. (2020). Characterizing the SBS polymer degradation within high content polymer modified asphalt using ATR-FTIR. Construction and Building Materials, 233, Article 117708. https://doi.org/10.1016/j.conbuildmat.2019.117708
Zenobi, M. C., Luengo, C. V., Avena, M. J., & Rueda, E. H. (2010). An ATR-FTIR study of different phosphonic acids adsorbed onto boehmite. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 75, 1283–1288. https://doi.org/10.1016/j.saa.2009.12.059
Downloads
Published
Issue
Section
License
La Editorial de la Universidad Nacional de La Pampa (EdUNLPam) exigirá a los/as autores/as la firma del siguiente documento:
La EdUNLPam lleva a cabo la publicación del artículo: (Título del Artículo) en SEMIÁRIDA Rev.Fac.Agron UNLPam ISSN 2362-4337 (impresa) ISSN 2408-4077 (en línea), del cual el/los abajo firmantes son autores de una o más partes. En el mismo acto, el/los autores entregan exclusivamente a la EdUNLPam todos sus derechos protegidos por las leyes de propiedad intelectual que rigen en la Argentina para reproducir, publicar, editar, fijar, comunicar y transmitir públicamente en cualquier formato o medio impreso o electrónico, inclusive internet, el artículo enviado a publicación e incluirlo en índices o bases de datos nacionales e internacionales. A cambio, la EdUNLPam entrega a los autores la autorización para la publicación o reimpresión con ines académicos y educativos en cualquier libro o medio de divulgación, con la sola obligación de citar el artículo original publicado en la EdUNLPam. Cada autor acuerda en que el material provisto a la EdUNLPam es un trabajo original, que no ha sido impreso o publicado en cualquier otro medio con anterioridad y que no vulnera derechos de terceros. El Primer autor tendrá la posibilidad de leer y corregir el artículo ya editado como “prueba de galera”, pero si el autor no devolviera esas correcciones de la prueba de galera dentro del tiempo especificado, el proceso de producción y publicación podrá proseguir sin la aprobación del autor. El/los autor/es no recibirán compensación monetaria de la EdUNLPam por el uso del material contenido en este artículo y asumen la responsabilidad de las opiniones vertidas en él.








.png)



22.png)



.jpg)




.jpg)
